





There is a wide variety of colors in minerals, but color is a the recognition criterium that is far from absolute. Specimens of different colors may represent the same mineral, such as quartz, which has several varieties of color that goes from clear colorless (rock crystal quartz), milky white, pink, purple (amethyst), yellow (citrine), etc ...

.. while specimens that have the same color can represent quite different minerals, like those minerals which all have a metallic luster and the color of gold: pyrite called "fool's gold" , chalcopyrite which is a copper ore, and real gold.

The brilliance of minerals is the aspect offered by their surface when reflecting light. There are two broad categories: - Metallic luster, shiny like metals, - Non-metallic luster which is described by terms such as . vitreous (like glass) . fat (as if the surface was coated with oil or grease) . adamantine (which reflects light like diamonds) . softwood (such as resin) . silky (like silk), etc ..
The streak of a mineral is the color it displays in finely powdered form. The streak maybe completely different from the color of the hand specimen. One of the simpliest ways of determining the strak of a mineral is to rub a specimen across a piece of unglazed porcelain known as a streak plate (provided that the hardness of the plate is greater than that of the mineral - see hardness). For example, hematite, a iron ore, has a black color on rupture but a reddish brown streak on the porcelain plate. Pyrite, has a golden yellow color but leaves a black streak.

Hematite (left) has a reddish-brown streak

Mineralogists have a relative hardness scale using ten common minerals, ranked from softest to hardest, from 1 to 10. This scale was constructed by the Austrian mineralogist Friedrich Mohs and is therefore called the Mohs scale . The hardness test is simple: Whoever is scratched by a Mohs standard is less hard than it, who scratches the standard is harder.
Density ($d$) of minerals is a property that characterizes a given mineral. Many minerals have a density that is about 2.7, so they are 2.7 times heavier than an equal volume of water. But some have a relatively low density, like salt that has a density of 2.1; others are at the other extreme, as galena (lead sulfide) with a density of 7.5 and gold having a density of 19.3.
Mesure:

- Measure the temperature - Fill the cylinder with water up to the mark. - Measure the mass of water + mass of the cylinder: $m_1$. - Empty. - Measure the mass of the mineral $m_2$. - Fill the cylinder ore and water to the same mark and measure the mass $M_3$. - Calculate the volumic mass $\rho$ of the ore and its density $d$ by using the following table:
Volumic mass of water

Calculation:
$m_3-m_2$ is the mass of the cylinder + the mass of the remaining water. $m_1-(m_3+m_2)$ is the mass of the water which takes the same volume $v$ than the mineral. $\rho_{mineral}$ $=$ $\frac{m_{mineral}}{V_{mineral}}$ $=$ $\frac{m_{mineral}}{v}$ $ =$ $\frac{m_2\rho_{water}}{m_1-(m_3+m_2)}$ $d_{mineral}$ $=$ $\frac{\rho_{mineral}}{\rho_{water}}$
Each mineral crystallizes in a given crystal system.
A crystal has a symmetry element, if it is generally invariant for this symmetry.
-Symmetry plane m

-Symmetry center -1

- Order 2 axis: 2

- Order 4 axis: 4
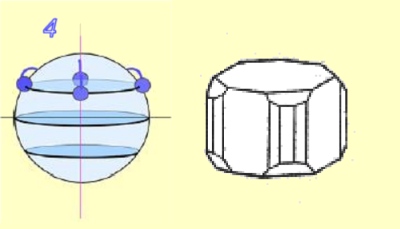
There are also order 3 and 6 axes: 3,6
→ Here more details on symetry
- The cubic system (isometric)
There are at least two order 3 axes.

Example: Fluorine
- The tetragonal system
There is one order 4 axis and no order 3 axis.

Example: Anastase
- The ortorhombic system
There are at least two order 2 axes or at least two symetry planes and no axis with another order.

Example: Sulfur
- The hexagonal system
There is one order 6 axis or one order 3 axis with perpendicular symetry plane.

Example: Mimetite
- The monoclinic system
There is one order 2 axis or/and one symetry plane.

Example: Euclase
- The triclinic system
There are no symetry axes or planes, but a symetry center may exist.
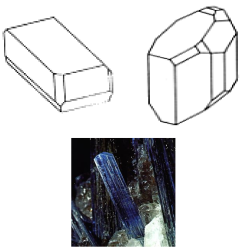
Example: Kyanite
The cleavage is a very important property of minerals. Minerals will easily break along cleavage planes. So if you hit most minerals (a good hammer!), you always get the same angles between the faces of the pieces, regardless of the size of the latter. For example, calcite has cleavage planes with 75 ° and 105 °.

However, if a quartz crystal, which is an mineral without cleavage planes, is broken, fragments are obtained with very irregular breakage. (as if a bottle was broken). Micas form phyllite leaves through their cleavage in a single plane.
Carbonate class minerals are chemically decomposed by acids, the chemical reaction gives off carbon dioxide bubbles, a phenomenon that describes effervescence (bubbling). According the carbonate mineral, this effervescence occurs on the mineral mass or even on dust, cold or hot.
Author: smi34770