







In acidic medium $1\; mol$ alcohol reacts with $1 \;mol$ of a carboxylic acid. The equilibrium constant is $K = 4$.
Determine the number of moles of the ester obtained at equilibrium.
Call $x$ the number of moles of ester obtained and then fill in the following table:
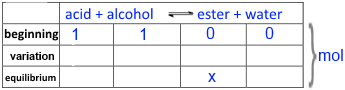
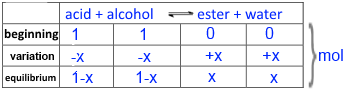
Introduce into the expression of the equilibrium constant K !
$4$ = $\frac{n_{ester}n_{eau}}{n_{acide}n_{alcool}}$= $\frac{x\cdot x}{(1-x) \cdot (1-x)}$
Solve the resulting equation!
$\frac{x\cdot x}{(1-x) \cdot (1-x)}=4$ $\frac{x^2}{(1-x)^2}=4$ $(\frac{x}{1-x})^2=4$ $\frac{x}{1-x}=2$ (mol numbers are positive, the negative solution -2 must therefore be rejected) $x=\frac{2}{3}$ Number of moles of ester obtained = $\frac{2}{3}$