







Search:

In ethanol sodium "dissolves" (= becomes a soluble substance, namely sodium ethanolate), evolving hydrogen.
The H atom has a different oxidation number in ethanol than in $H_2$:
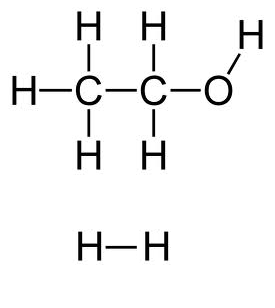
Reminder: Oxidation numbers are determined by attributing to the more electronegative atoms all electrons of the covalent bond, and are equal to the dummy load thus obtained.
Ethanol $\Rightarrow$ ethanolate and hydrogen: $2CH_3CH_2OH$ $+$ $2e^-$ $\rightarrow$ $ 2CH_3CH_2O^-$ $+$ $H_2$
$2CH_3CH_2OH$ $+$ $2e^-$ $\rightarrow$ $ CH_3CH_2O^-$ $+$ $H_2$
$2Na$ $-$ $2e^-$ $\rightarrow$ $2Na^+$ | $\cdot 2$
 $2CH_3CH_2OH$ $+$ $2Na$ $\rightarrow$ $ 2CH_3CH_2O^-$ $ +$ $2Na^+$ $+$ $H_2$
This reaction is sometimes interpreted as due to an "acidic" character ethanol.
It is initiated by a first acid dissociation of the molecule followed by reaction of the ion $H^+$ formed with sodium::
$2CH_3CH_2OH$ $\rightarrow$ $ 2CH_3CH_2O^-$ $+$ $2H^+$
then:
$2H^+$ $+$ $2e^-$ $\rightarrow$ $ H_2$
$2Na$ $-$ $2e^-$ $\rightarrow$ $2Na^+$
$2CH_3CH_2OH$ $+$ $2Na$ $\rightarrow$ $ 2CH_3CH_2O^-$ $ +$ $2Na^+$ $+$ $H_2$
This reaction is sometimes interpreted as due to an "acidic" character ethanol.
It is initiated by a first acid dissociation of the molecule followed by reaction of the ion $H^+$ formed with sodium::
$2CH_3CH_2OH$ $\rightarrow$ $ 2CH_3CH_2O^-$ $+$ $2H^+$
then:
$2H^+$ $+$ $2e^-$ $\rightarrow$ $ H_2$
$2Na$ $-$ $2e^-$ $\rightarrow$ $2Na^+$
 $2H^+$ $+$ $2Na$ $\rightarrow$ $ 2Na^+$ $+$ $H_2$
Sodium ethanolate such formed is colorless and soluble in ethanol:
$2H^+$ $+$ $2Na$ $\rightarrow$ $ 2Na^+$ $+$ $H_2$
Sodium ethanolate such formed is colorless and soluble in ethanol: