







Search:

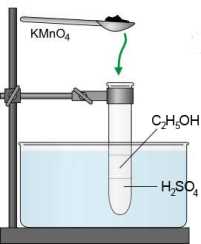
Ethanol $\Rightarrow$ ethanal: $CH_3CH_2OH$ $-$ $2e^-$ $\rightarrow$ $ CH_3CHO$ $+$ $2H^+$ Ethanal $\Rightarrow$ ethanoic (acetic) acid: $CH_3CHO$ $-$ $2e^-$ $+$ $H_2O$ $\rightarrow$ $ CH_3COOH$ $+$ $2H^+$
- First step:
Ethanol $\Rightarrow$ ethanal:
$CH_3CH_2OH$ $-$ $2e^-$ $\rightarrow$ $ CH_3CHO$ $+$ $2H^+$ | $\cdot 5$
$MnO_4^-$ $+$ $5e^-$ $+$ $8H^+$ $\rightarrow$ $ Mn^{2+}$ $+$ $4H_2O$ | $\cdot 2$
 $5CH_3CH_2OH$ $+$ $2MnO_4^-$ $ +$ $6H^+$ $\rightarrow$ $ 5CH_3CHO$ $ + $ $2Mn^{2+}$ $+$ $8H_2O$
$5CH_3CH_2OH$ $+$ $2MnO_4^-$ $ +$ $6H^+$ $\rightarrow$ $ 5CH_3CHO$ $ + $ $2Mn^{2+}$ $+$ $8H_2O$
- Second step: ethanal $\Rightarrow$ ethanoic (acetic) acid:
$CH_3CHO$ $-$ $2e^-$ $+$ $H_2O$ $\rightarrow$ $ CH_3COOH$ $+$ $2H^+$ | $\cdot 5$
$MnO_4^-$ $+$ $5e^-$ $+$ $8H^+$ $\rightarrow$ $ Mn^{2+}$ $+$ $4H_2O$ | $\cdot 2$
 $5CH_3CHO$ $+$ $2MnO_4^-$ $ +$ $6H^+$ $\rightarrow$ $ 5CH_3COOH$ $ +$ $ 2Mn^{2+}$ $+$ $3H_2O$
$5CH_3CHO$ $+$ $2MnO_4^-$ $ +$ $6H^+$ $\rightarrow$ $ 5CH_3COOH$ $ +$ $ 2Mn^{2+}$ $+$ $3H_2O$
A color change from purple $(MnO_4^-)$ to palepink $(Mn^{2+})$ is observed !