







Consider a $0.050\;M$ solution of the weak base pyridine ($C_5H_5N$ , $K_b$ $=$ $1.7 \cdot 10^{-4}$)
$C_5H_5N$ $+$ $H_2O$  $HC_5H_5N^+$ $+$ $OH^-$
$HC_5H_5N^+$ $+$ $OH^-$
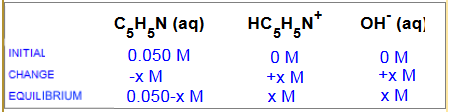
Which of following expressions represents the correct $K_b$ after substitution?
a) $1.7 \cdot 10^{-4}$ $=$ $\frac{(0,050-x)x}{x}$ b)$ 1.7 \cdot 10^{-4}$ $=$ $\frac{x^2}{0,050}$ c) $1.7 \cdot 10^{-4}$ $=$ $\frac{0,050-x}{x^2}$ d) $1.7 \cdot 10^{-4}$ $=$ $\frac{x^2}{0,050-x}$
Correct answer: d) $K_b$ $=$ $1.7 \cdot 10^{-4}$ $=$ $ \frac{x^2}{0,050-x}$ Resolving this equation we find: $[OH^-]$ $=$ $x$ $=$ $1,3\cdot 10^{-2} \;M$