







Consider a $0.050\;M$ solution of the weak base pyridine ($C_5H_5N$ , $K_b$ $=$ $1.7 \cdot 10^{-4}$)
$C_5H_5N$ $+$ $H_2O$  $HC_5H_5N^+$ $+$ $OH^-$
$HC_5H_5N^+$ $+$ $OH^-$
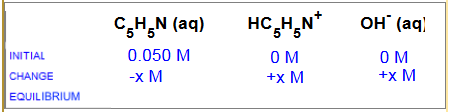
What are the molar concentrations at equilibrium of each species?
a) $x\;M$, $(0.050-x)\;M$, $x\;M$ b) $(0.050-x)\;M$, $x\;M$, $x\;M$ c) $x\;M$, $x\;M$, $x\;M$ d) $(0.050-x)\;M$, $(0.050+x)\;M$, $x\;M$
Correct answer: b) $(0.050-x)\;M$, $x\;M$, $x\;M$