







Search:
In the "electron gas" of the metals, the electrons occupy energy levels belonging to the whole metal and very close to oneanother. Therefore they ensure the cohesion of the metal, although they remain very mobile:
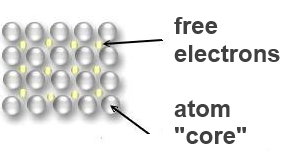
The mobility of these electrons explains the properties of the metals:
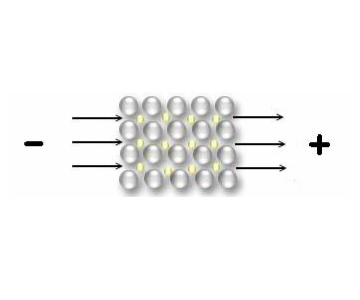
The electrons are almost free in the metal, so they move immediately when a tension is applied :
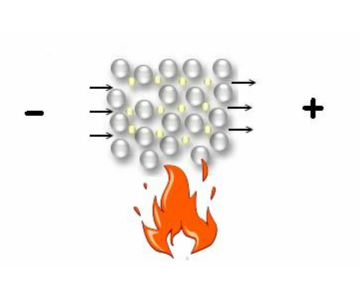
Heat exerces a twofold influence on a metal: The mobility of the electrons explains why heat spreads rapidly in every direction, on the other hand, the random movement of the atom cores in the metal impedes the ordered movement of electrons when they are subject to an external tension: the electrical conductivity diminishes with increasing temperature.

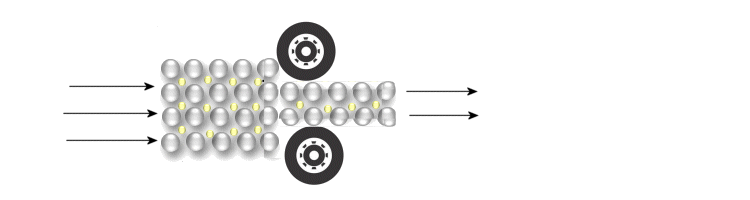
When a metal is plastically deformed, the layers of atom cores slip past another without disturbing the electron gas. Metals can be deformed and flattened to metal sheets.
A metal can be stretched easily to fine wires.
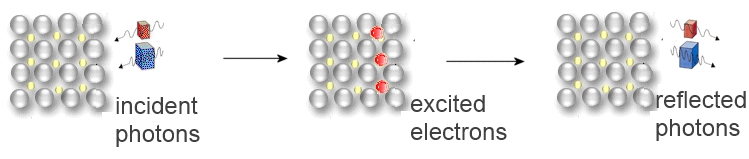
When the surface of a metal is well polished, all incident photons excite the outer electrons of the metal. Falling back to their normal energy level, these electrons emit photons , and that explains the metallic luster. When the metal is powdered, the photons moving in all directions are mutually canceled like waves on a pebbled beach. Metal powder has often a black colour.
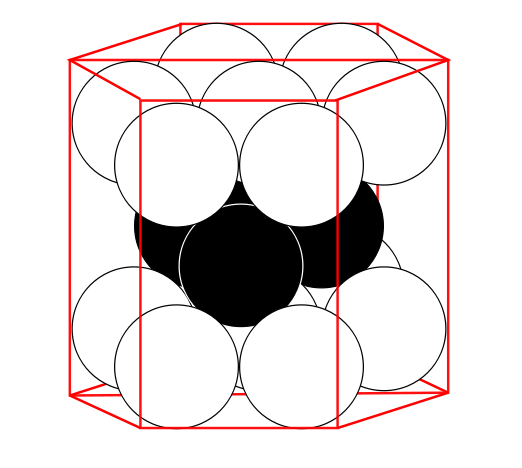
The atom cores are almost unaffected by the electrons. Therefore they adopt frequently the most compact assembly with hexagonal geometry.