B- is a strong base eg HO- or CH3O- or CH3CH2O- ...
(As opposed to strong nucleophiles, strong bases have small negatively charged atoms such that they can more strongly attract the small H+ ion ).
X is an outgoing group that has a strong tendency to associate an electronic doublet eg Cl, Br, I, OH ....
(Here the essential thing is that the formed ion be stable which is all the more the case that it is big and that its charge is "diluted")
The speed of this reaction depends on both the concentration of the base and that of the substrate (bimolecular reaction)
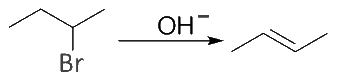
Example:
B- is a strong base eg HO- or CH3O- or CH3CH2O- ...
(As opposed to strong nucleophiles, strong bases have small negatively charged atoms such that they can more strongly attract the small H+ ion ).
X is an outgoing group that has a strong tendency to associate an electronic doublet eg Cl, Br, I, OH ....
(Here the essential thing is that the formed ion be stable which is all the more the case that it is big and that its charge is "diluted")
The speed of this reaction depends on both the concentration of the base and that of the substrate (bimolecular reaction)
Example:

 B is a weak base eg H2O or CH3CH2OH
X is an outgoing group that has a strong tendency to associate an electronic doublet eg Cl, Br, I, OH ....
X is often linked to a tertiary C atom whose carbocation formed is particularly stable because of the positive inductive effects of the alkyl groups attached thereto.
The speed of this reaction depends only on that of the substrate whose cation dissociation rate is slow (monomolecular reaction)
Example:
B is a weak base eg H2O or CH3CH2OH
X is an outgoing group that has a strong tendency to associate an electronic doublet eg Cl, Br, I, OH ....
X is often linked to a tertiary C atom whose carbocation formed is particularly stable because of the positive inductive effects of the alkyl groups attached thereto.
The speed of this reaction depends only on that of the substrate whose cation dissociation rate is slow (monomolecular reaction)
Example:


Alexander Mikhaylovich Zaitsev
The alkene formed in greatest amount is the one that corresponds to removal of the hydrogen from the ß-carbon having the fewest hydrogen substituents.
Example: