Often: Weak oxidizing agents: Position 1 : $ - CHO $ → $ -COOH $ Strong oxidizing agents: Position 1 : $ -CHO $ → $ -COOH $ and position 6 : $ -CH_2OH $ → $ -COOH $ ($ \omega $-oxidation) Strong oxidizing agents: and protected position: 1 Position 6 : $ -CH_2OH $ → $ -COOH $ ($ \omega $-oxidation)
Examples
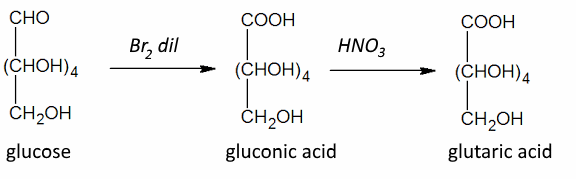
Gluconic acid is naturally present in many fruits. It has many uses in dietetics, medicine and industry!
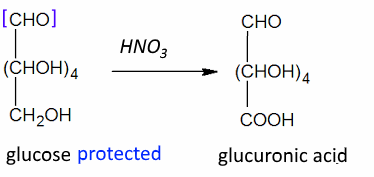
Such protection is often in the form of acetals $ CHO $ → $ R^1-O-CH-O-R^2$
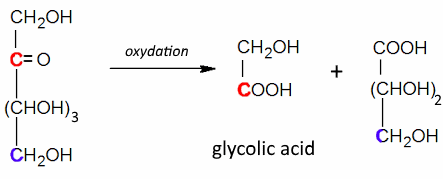
Often: Low Oxidizers: Rupture between positions 2 and 3 and oxidation of these positions to $ -COOH $