







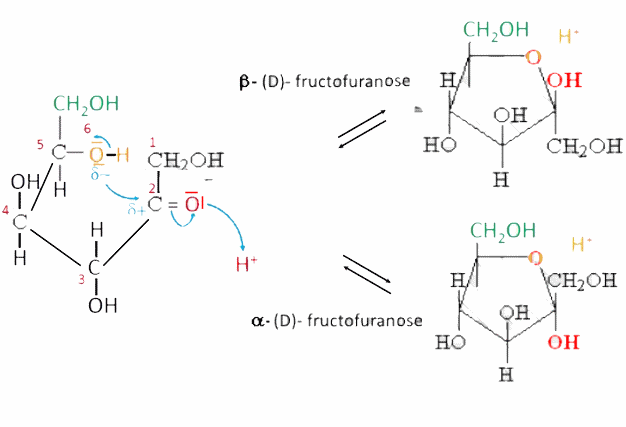
In aqueous solution, the nucleophilic attack of the oxygen of the penultimate hydroxide group on the carbonyl carbon which leads to the structure $ -OC (OH) - $ is a Hemiacetization :

- The reaction produces a heterocycle (one $ -O- $ bond) - Depending on whether the ring has 5 or 6 atoms, it is related to furan or pyran:

- A new asymmetric C atom is formed ( $ C^* $ ). - The $ \alpha $ enantiomer has its hydroxide group below the ring, ie. on the opposite side to the $ - CH_2OH $ group. - The enantiomer $ \beta $ has its hydroxide group above the ring, ie. on the same side as the $ - CH_2OH $ terminal group.

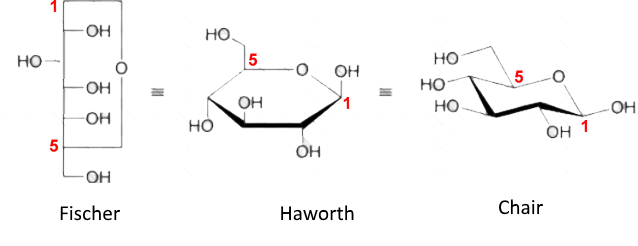
The representations of the cyclic forms can vary, the right one (chair shape) of $ \beta $-D-glucopyranose is closest to reality.