Concentrations: molality and molar fraction:
Solute, solvent and solution
The solute ($so$) is the dissolved species, the solvent (sl) is the liquid in which this species is dissolved and the solution (S) is the mixture of the two. The mass of the solution is always equal to the mass of the solvent + the mass of the solute.
$m_S$=$m_{so}+m_{sl}$
Such equality is not always true for volumes!
Molar fraction of solute ($X_ {so}$)
The molar fraction of the solute is the ratio of the number of moles of solute to the total number of moles of the mixture. In the case of a single solute, the total number of moles of the mixture is the sum of the number of moles of solute and solvent:
$X_{so}$=$\frac{n_{so}}{n_{so}+n_{sl}}$
Molality of solute ($\mu_{so}$)
The molarity of the solute is the ratio of the number of moles of solute to the mass of the solvent.
$\mu_{so}$=$\frac{n_{so}}{m_{sl}}$
if $m_{sl}$ is expressed kg
On putting allthis together:
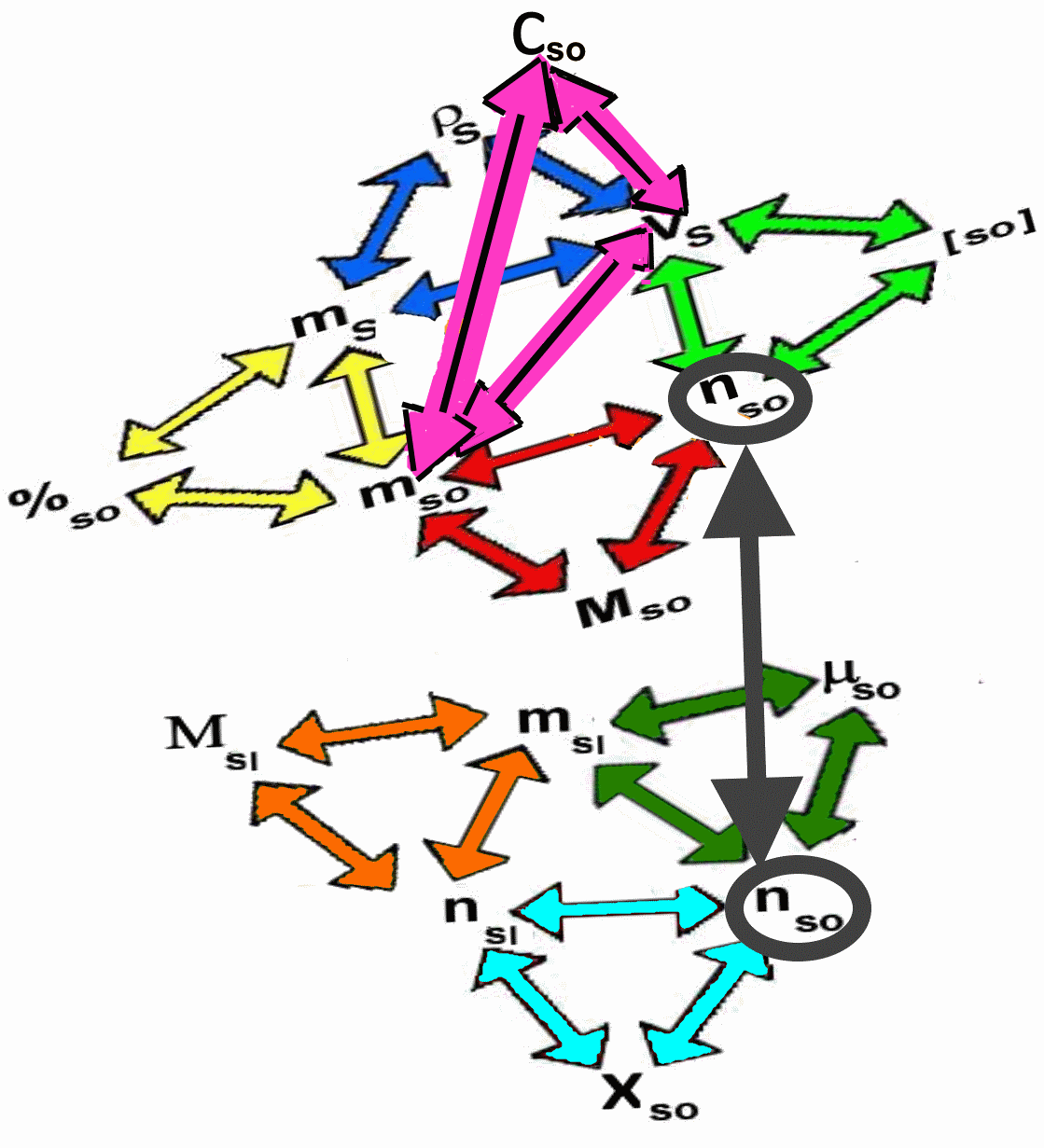
The "lattice"
The following "lattice" summarizes the relationships between the previous quantities:
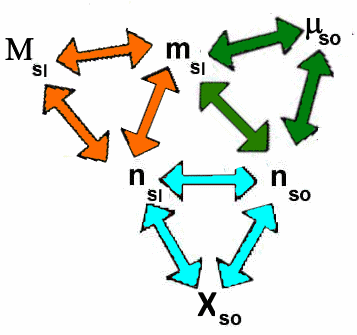
Each triangle symbolizes one of the preceding definitions. For example, if we know $\mu_{so} $ and $n_{so} $, we compute $m_{sl} $ by the green triangle and then $n_{sl} $ by the orange triangle (knowing the molar mass of the solvent), then again $X_{so} $ by the blue triangle!