








Vladimir Markovnikov 1837-1904
Stability of carbocations increases with the inductive effect of the alkyl groups attached to carbon carrying the positive charge.
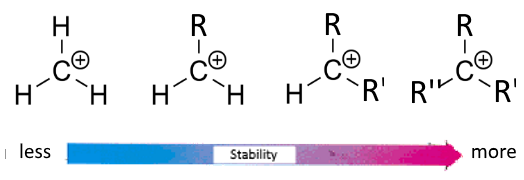
Pushing electrons to the charge center, the inductive effect partially fills the positive charge and makes it less virulent, thus more stable:
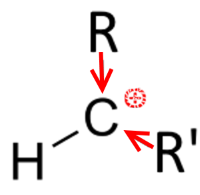
This allows to predict the asymmetric addition on an alkene, for example:
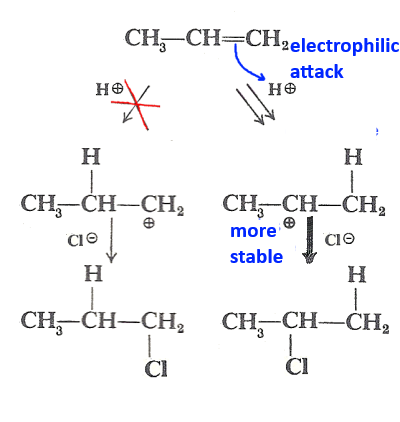
In the addition of hydrogen halide to an asymmetric alkene, the hydrogen ion is fixed on the carbon which carries most of hydrogen atoms. or better:
In the addition of an ionic compound of the H - X type on a carbon-carbon double bond, the major product is the one derived from the more stable carbocation formed upon electrophilic attack.