





 Adolphe Wurtz 1817-1884
Adolphe Wurtz 1817-1884
Is added to a dry flask - a solution of an alkyl halide $RX $ in an inert solvent - metallic sodium $Na$ or lithium $Li$ The reaction is immediate: $2R-X+2Na\rightarrow R-R +2NaX$ $2R-X+2Li\rightarrow R-R +2LiX$ The coupling produces a symmetrical molecule!

In the flask there is a chloroethane solution in an inert solvent containing a few pieces of lithium The reaction product gas is collected in a gas holder. One liter of this gas is collected under normal conditions in an inflatable balloon and weighed:
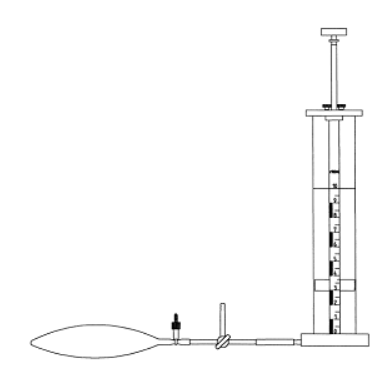
We find (normal conditions!) $\rho= 2,60 \; \frac{g}{L}$ Calculate the molar mass of the product and confirm the coupling reaction! →
Write the products of the following reactions of alkyl halides with sodium: - 2-iodopropane →
- 1,2-dichloroethane →
- Dichlorocyclopropane →