







Search:

|
1. $H^+$ |
$2CrO_4^{2-}+2H^+$ $\rightarrow$ $Cr_2O_7^{2-}+H_2O$ - dichromate ion - orange (There is no change in the E.O. of chromium) |
|
2. Reductants in acidic medium ($SO_2$, isopropanol, $H_2S$, ascorbic acid) |
$2CrO_4^{2-}$ $+$ $8H^+$ $+$ $3e^-$ $\rightarrow$ $Cr^{3+}$ $+$ $4H_2O$ - (-> Chromium(III) ion (green)) |
|
2. Peroxyde(in the presence of $H_2SO_4$ dil. and ether) |
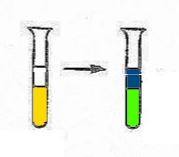
$2CrO_4^{2-}$ $+$ $2H^+$ $+$ $2H_2O_2$ $\rightarrow$ $CrO_5$ $+$ $3H_2O$ - $CrO_5$ is blue and stable in ether - In the water, $H_2O_2$ reduces $CrO_4^{2-}$ to $Cr^{3+}$ (To test with a dilute solution of chromate. The reaction is used, backwards, in the test for the peroxo bond, eg in ether) |
|
3. Diphenylcarbazide (in acid medium) 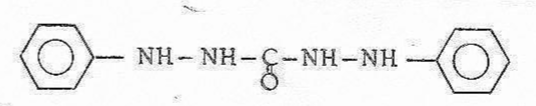
|
|
Formation of a purple coloring, trace reaction! (To test with a very dilute solution of chromate containing many HCl) |
|
4. $Ag^+$ (Silver nitrate) |
$2CrO_4^{2-}$ $+$ $2Ag^+$ $\rightarrow$ $Ag_2CrO_4$ - Silver chromate - Red precipitate - Soluble in $HNO_3$ |
|
4. $Ba^2+$ (Barium chloride) |
$CrO_4^{2-}$ $+$ $Ba^{2+}$ $\rightarrow$ $BaCrO_4$ - Barium chromate - Lemon yellow precipitate - Soluble in $HCl$ |
|
4. $Pb^2+$ (Lead nitrate) |
$CrO_4^{2-}$ $+$ $Pb^{2+}$ $\rightarrow$ $PbCrO_4$ - Lead chromate - Yellow precipitate - Soluble in $HNO_3$ and $KOH$ |