







Search:
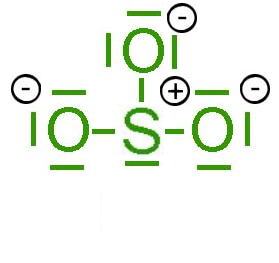
|
1. $Ba^{2+}$ (Barium chloride) |
$SO_3^{2-}+Ba^{2+}$ $\longrightarrow$ $BaSO_3$ - White precipitate - Soluble in $HCl$ |
|
2. $Ag^+$ (Silver nitrate) |
|
$SO_3^{2-}+2Ag^+$ $\longrightarrow$ $Ag_2SO_3$ - White precipitate - Soluble in $HCl$ |
|
3. $H_2SO_4$ conc. or dil. |
|
$SO_3^{2-}+2H^+$ $\longrightarrow$ $H_2O+SO_2$ - smell ! |
|
4. $MnO_4^-$ (Permanganate in acid medium) |
|
$5H_2SO_3^{2-}+2MnO_4^-$ $\longrightarrow$ $5SO_4^{2-}+2Mn^{2+}+4H^++3H_2O$ - permanganate is discolored |
|
5. $I_2$ ((Iodine in acid medium) |
|
$H_2SO_3^{2-}+I_2+H_2O$ $\longrightarrow$ $SO_4^{2-}+2I^-+H^+$ - iodiner is discolored |