








The colorless gas produced is ethene: $\ce {C_2H_5OH ->[Al_2O_3] CH_2=CH_2 +H_2O} $ Instead of aluminium oxide we could as well take $170^oC$ sulfuric acid : $\ce {C_2H_5OH ->[{H_2SO_4}][{170^oC}] CH_2=CH_2 +H_2O} $ Ethene causes bromine water discoloration:
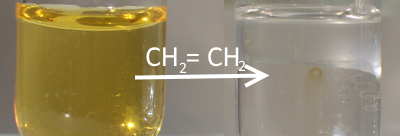
$\ce {CH_2=CH_2 +Br_2 -> CH_2Br-CH_2Br} $ Dibromo-1,2-ethane is colorless