






If the vapour of a substance occupies a given space, it has at equilibrium only one vapour pressure, regardless of the source of its molecules.
The following diagram shows the saturated vapour pressure of water:
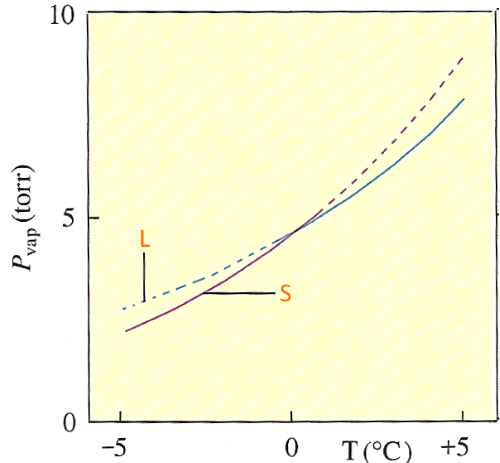
$L$ = liquid water $S$ = ice The shaded areas are extrapolated!
In the case of the substance water, $0^oC $ is the temperature where the vapour pressure of the saturating water and ice are the same. In general: - If liquid and solid phase of a substance are present at a temperature higher than the temperature where saturation vapour pressures are equal, the solid releases the vapour at a higher pressure than the saturated vapour pressure of the liquid (see diagram). As there can be only one pressure vapour of the substance, the liquid will try to reduce pressure by capturing molecules released by the solid and this as long as there's solid in presence. That is the fusion! - If liquid and solid phase of a substance are present at a temperature below the temperature where saturation vapour pressures are equal, the liquid releases vapour at a higher pressure than the saturation vapour pressure (see diagram). As there can be only one vapour pressure of the substance, the solid will try to reduce pressure by capturing molecules released by the liquid and this while there is liquid present. That is solidification! - If liquid and solid phase of a substance are in presence at the temperature where saturation vapour pressures are equal, saturation vapour pressures are the same and the two substances can coexist with vapour. This temperature is the melting point.