





The molecules of a substance do not move all at the same speed. Each molecule has its own kinetic energy: $E_c$ $=$ $\frac{1}{2}mv^2$ where $m$ is its mass $v$ its speed

Ludwig Boltzmann (1844-1906)
Boltzmann found that at a given temperature, the molecule speeds of a certain quantity of substance are distributed according to a characteristic curve:
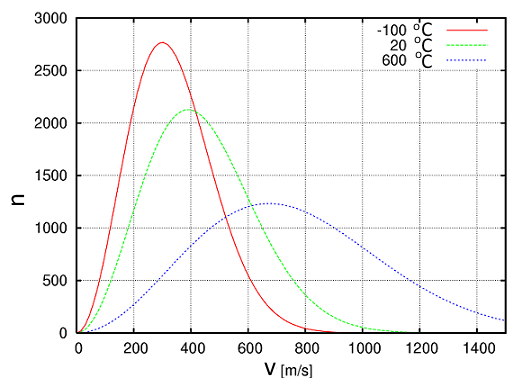
oł: $v$ is the speed of one molecule $n$ the number of molecules having that speed
- In fact, Boltzmann gave an intuitive meaning of this mysterious physical parameter which we call temperature: Temperature is a quantity proportional to the average kinetic energy of molecules! - At a higher temperature, not just the average kinetic energy increases, but the distribution curve flattens!
In a liquid molecules are retained by the mutual attraction (dipoles or van der Waals attractions). On the other hand, according to Boltzmann, their speeds can differ greatly. Consider our liquid in a closed container above which air was evacuated:
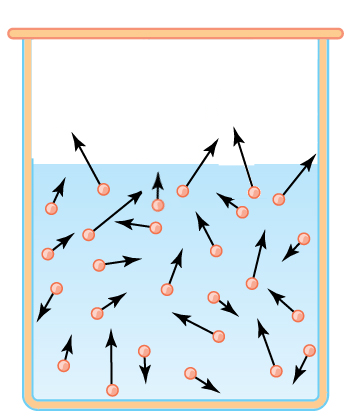
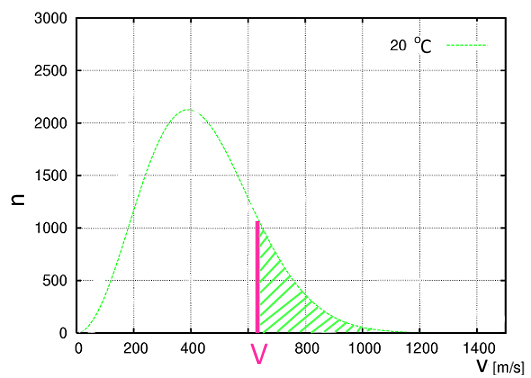
To escape from the liquid, at a given temperature, the molecules must exceed a certain speed $V$ . There will certainly exist such molecules! (hatched area).
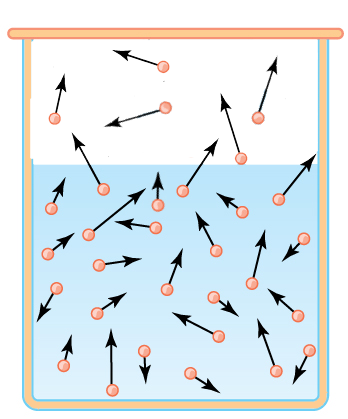
In the gaseous phase the Boltzmann distribution of velocities is quickly established. Molecules will be recaptured by the liquid:
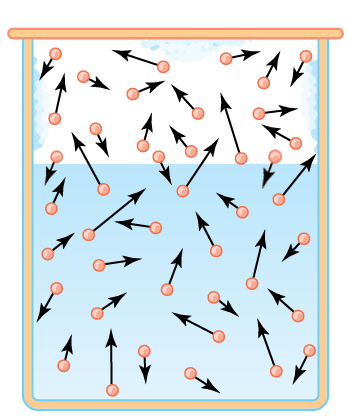
It results from there an equilibrium!
Let's apply the law of Guldberg and Waage (Special case of heterogeneous equilibrium): $P_o$ $=$ $K$ The vapour pressure $P_o$ must remain constant from that moment on:
When a substance is in equilibrium between a gas phase and a liquid phase, and it is pure in both phases, the pressure in the gas phase is constant at equilibrium and at a certain temperature: $P_o$ $ =$ $K$ $P_o$ is a constant for a given temperature, called "(Saturation) Vapour Pressure" of the substance in the gaseous phase.
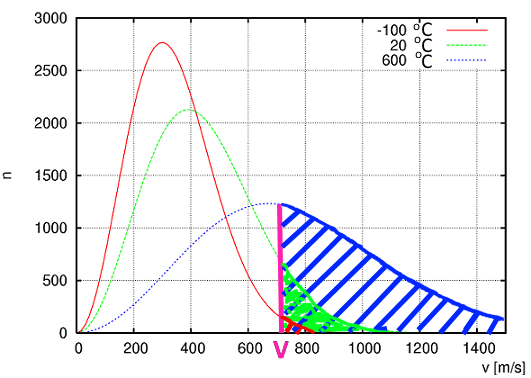
At higher temperature, the proportion of molecules that reach the speed $ V $ where they can escape the liquid phase is higher (hatched area )
(Saturation) vapour pressure increases (quickly) with temperature.
If the attractions between molecules are weak, more molecules can escape at a given temperature, so the vapour pressure is higher This is particularly the case for non-polar substances (hydrocarbons, substances with symmetrical molecules, etc ..)
Non-polar (or slightly polar) substances have high (saturation) vapor pressures.

Diagram of (saturation) vapor pressure depending on the temperature: 1 Diethyl ether (weakly polar) 2 Ethanol (polar) 3 Water (highly polar)