





The nitrogen reacts with the hydrogen to form ammonia.
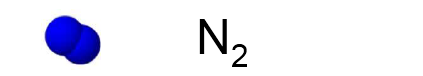
One molecule of nitrogen

One molecule of hydrogen

One molecule of ammonia
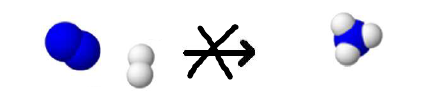
Impossible because there are too much nitrogen atoms and too little hydrogen atoms!
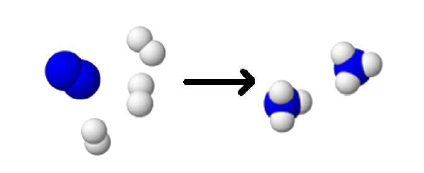
Correct !
Balanced equation :
$N_2$ $+$ $3H_2$ $\longrightarrow$ $2NH_3$
- The correct formulas of the reactants separated by + are written to the left of the arrow. - The correct formulas of the products separated by + are written to the right of the arrow. - Looking at the reactant or product with the most atoms of various kinds, we attribute (temporarily) the coefficient 1 to this substance - We try to write the coefficients of the other substances so as to have the same number of atoms of each species left and rightof the arrow. - If necessary, all is multiplied by an appropriate factor.