







Search:

Arsenic
| Arsenic(III) |
|
1. $H_2S$ |
|
$2AsO_3^{3-}$ $+$ $3S^{2-}$ $+$ $12H^+$ $\longrightarrow$ $6H_2O$ $+$ $As_2S_3$ - Canary yellow precipitate - Soluble in $(NH_4) _2S$ - Soluble in $KOH$ - Soluble in $NH_3$ - Soluble in $(NH_4)_2CO_3$ - Previous cases: reprecipitation $HCl$ or diluted $H_2SO_4$ - Insoluble in $HCl$ even fuming |
|
2. $AgNO_3$ |
|
$AsO_3^{3-}$ $+$ $3Ag^+$ $\longrightarrow$ $Ag_3AsO_3$ - Yellow precipitate - Soluble in $HNO_3$ - Soluble in $NH_3$ |
|
3. Magnesium mixture 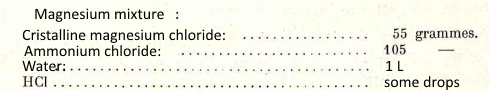
|
|
No precipitation |
| Arsenic(V) |
|
1. $H_2S$ |
|
- In cold: no precipitation - In hot: reduction of As (III) and reaction: $AsO_4^{3-}$ $+$ $S^{2-}$ $+$ $2H^+$ $\longrightarrow$ $AsO_3^{3-}$ $+$ $H_2O+S^0$ $2AsO_3^{3-}$ $+$ $3S^{2-}$ $+$ $12H^+$ $\longrightarrow$ $6H_2O$ $+$ $As_2S_3$ |
|
2. $AgNO_3$ |
|
$AsO_4^{3-}$ $+$ $3Ag^+$ $\longrightarrow$ $Ag_3AsO_4$ - Chocolate brown precipitate - Soluble in $HNO_3$ - Soluble in $NH_3$ |
|
3. Mixture magnésienne 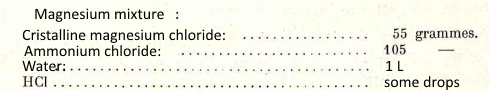
|
|
$AsO_4^{3-}$ $+$ $Mg^{2+}$ $+$ $NH_4^+$ $\longrightarrow$ $MgNH_4AsO_4$ - White precipitate - Instantly formed |