





The following images were taken from the presentation by → Dimitri Taits
The ancient Egyptians knew how to make soap:
To do this, they took palm oil

they mixed with ash containing 25% potash K 2 CO 3

and then heating the mixture to a smooth paste, soap.


30 ml of olive oil are added to a flask.
The images were taken from the experiment presented by → Coollege
Palm oil contains glyceryl palmitate ester ( propane-1,2,3-triyl hexadecanoate)
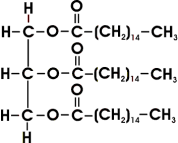
Hydrolysis of this ester is limited to an equilibrium
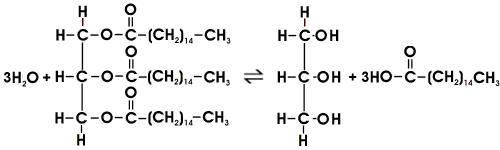
Reacting with the palmitic acid, sodium hydroxide removes it and shifts the equilibrium to the right.
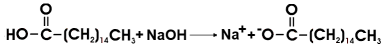
Sodium palmitate is partially soluble in water. The addition of sodium ions by leaching shifts the precipitation equilibrium to the right.

Palm oil soap is palmitate sodium salt (partially soluble in water) mixed with more or less glycerine.
To make soaps, we can replace palm oil with another lipid such as olive oil or stearin, NaOH may be replaced by KOH or same carbonates such as Na2CO3 and K2CO3