





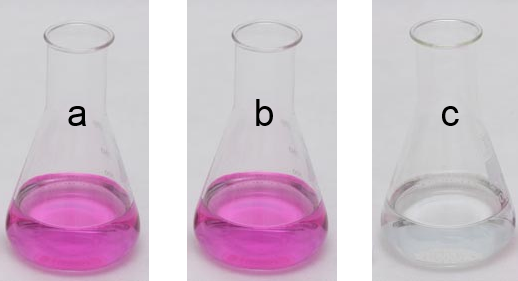
The Erlenmeyer a contains a solution of ethylamine with some phenolphthalein. The $OH^-$ ions liberated by the equilibrium $CH_3CH_2NH_2 (g)$ $+$ $H_2O (g)$ $\leftrightarrows$ $ CH_3CH_2NH_3^+$ $+ $ $OH^-$ color phenolphthalein in dark pink.
In b, few hydrochloric acid was added, the phenolphthalein kept its color.
In c , an excess of hydrochloric acid was added, the phenolphthalein became colorless.
In b , there are not enough $H_3O^+$ ions from hydrochloric acid to neutralize all the $OH^-$ ions from the amine:
$H_3O^+$ $+$ $OH^-$ $\rightarrow $ $2H_2O$
By adding the "spectator" ions : $H_3O^+$ $+$ $Cl^-$ $+$ $CH_3CH_2NH_3^+$ $+$ $OH^-$ $\rightarrow$ $2H_2O$ $+$ $CH_3CH_2NH_3^+$ $+$ $Cl^-$ Since there is a rest of $OH^-$ ions, phenolphthalein keeps its color. In c , all $OH^-$ ions were neutralized, phenolphthalein is discolored.

Left:
$Fe^{2+}$ $+$ $2OH^-$ $\rightarrow$ $ Fe^{2+}(OH^-)_2$
By adding the "spectator" ions : $Fe^{2+}$ $+$ $2Cl^-$ $+$ $2CH_3CH_2NH_3^+$ $+$ $2OH^-$ $\rightarrow$ $ Fe^{2+}(OH^-)_2$ $+$ $2CH_3CH_2NH_3^+$ $+$ $2Cl^- $ Iron(II) hydroxide is a dirty green precipitate. À droite:
$Fe^{3+}$ $+$ $3OH^-$ $\rightarrow$ $ Fe^{3+}(OH^-)_3$
By adding the "spectator" ions : $Fe^{3+}$ $+$ $3Cl^-$ $+$ $3CH_3CH_2NH_3^+$ $+$ $3OH^-$ $\rightarrow$ $ Fe^{3+}(OH^-)_3$ $+$ $3CH_3CH_2NH_3^+$ $+$ $3Cl^- $ Iron(III) hydroxide is an ocher-brown precipitate.