







Search:

Nickel(II) chloride
|
1. $H_2S$ |
|
No precipitation in acid medium |
|
2. $(NH_4)_2S$ |
|
$Ni^{2+}$ $+$ $S^{2-}$ $\longrightarrow$ $NiS$ - Black precipitate - Soluble in $HCl$ - Soluble $HCl/HNO_3$ , $S_8$ glomerulus ! |
|
3. $KOH$ |
|
$Ni^{2+}$ $+$ $2OH^-$ $\longrightarrow$ $Ni(OH)_2$ - Green gelatinous precipitate - Soluble in dilute mineral acids - Insoluble in excess reagent - Is oxidized in the presence of oxidants, f.i. $Br_2$ : $Ni(OH)_2$ $+$ $Br_2$ $+$ $2OH^-$ $\longrightarrow$ $2Br^-$ $+$ $2Ni(OH)_3$ - Black precipitate |
|
4. $NH_3$ |
|
$Ni^{2+}+2OH^- \longrightarrow Ni(OH)_2$ - Green precipitate - With an excess of $NH_3$ , there is formation of a complex: $Ni(OH)_2 ..$ $\longrightarrow$ $ ..[Ni^{II}(NH_3)_6]^{2+}$ - Hexamminenickel(II), soluble, purple |
|
5. $KCN$ |
|
$Ni^{2+}$ $+$ $2CN^-$ $\longrightarrow$ $Ni(CN)_2$ - Green precipitate - With an excess of $CN^-$ , there is formation of a complex: $Ni(CN)_2 ..\longrightarrow ..[Ni^{II}(CN_4)]^{2-}$ - Tetracyanonickelate(II), soluble, yellow - The reaction masks all other reagents to $Ni^{2+}$ |
|
6. Dimethylglyoxime |
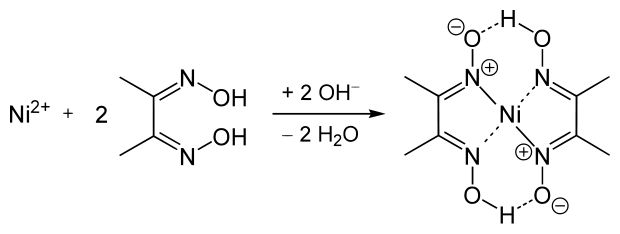
- Chelate bis(diméthylglyoximato)nickel, raspberry-red - Soluble in dilute mineral acids - Insoluble in acetic acid - The ammonia medium promotes the precipitation - Avoid mineral acids and oxidizing |