





$100 \;mL$ $0.1\; M$ acetic acid were mixed with $300\; mL$ of $0.1\; M$ sodium acetate (well known acetic buffer )
a) Prepare for the mixture 5 equations between molarities at equilibrium:
$[Na^+]$, $[H_3O^+]$, $[CH_3COO^-]$, $[CH_3COOH]$ and $[OH ^ -]$
(the electroneutrality condition, two conditions of conservation of matter, an equilibrium condition and the ionic product of water)
b) Show that the resolution of this system leads to the equation
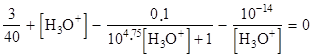 c) Solve this equation using → this .
c) Solve this equation using → this .