





Here are some examples that show the enormous impact of the $pH$ on nature and everyday life:
- Acid rain
 On the origin of acid rain, there are mainly sulfur oxides and nitrogen oxides produced by industry, power plants, heating and transport. ... These pollutants react in the atmosphere with oxygen to acidify rainwater. The low $pH$ of rain damages vegetation.
- Natural waters
On the origin of acid rain, there are mainly sulfur oxides and nitrogen oxides produced by industry, power plants, heating and transport. ... These pollutants react in the atmosphere with oxygen to acidify rainwater. The low $pH$ of rain damages vegetation.
- Natural waters
 Waters through bogs and rain forests ("black water") have a low pH.
Waters through bogs and rain forests ("black water") have a low pH.
 Microorganisms contained therein are worse fish food than those present in other natural waters.
- Cultures
Microorganisms contained therein are worse fish food than those present in other natural waters.
- Cultures
 In acidic soils metal cations concentrations increase to become harmful to plants.
Under basic conditions (alkaline), the solubility of useful cations decreases causing chlorosis.
In acidic soils metal cations concentrations increase to become harmful to plants.
Under basic conditions (alkaline), the solubility of useful cations decreases causing chlorosis.
 In most cases a favorable compromise is reached between $pH$ 5.8 and 6.5 .
- Health
In most cases a favorable compromise is reached between $pH$ 5.8 and 6.5 .
- Health
 Food influences the $pH$ of blood. A low $pH$ eg from the consumption of too much meat, fish, eggs, grains, sugar can
- make skin, hair, nails and bones vulnerable
- deteriorate the gut
- promote candidiasis (skin diseases caused by yeasts)
- facilitate allergies
- make nervous system more excitable , cause sciatica
- cause anxiety or depressions
- be responsible for muscle cramps
- make it more susceptible to infections
- cause chronic fatigue
Many of these ailments stem from the fact that too high acidity blocks essential minerals.
- Teeth
Food influences the $pH$ of blood. A low $pH$ eg from the consumption of too much meat, fish, eggs, grains, sugar can
- make skin, hair, nails and bones vulnerable
- deteriorate the gut
- promote candidiasis (skin diseases caused by yeasts)
- facilitate allergies
- make nervous system more excitable , cause sciatica
- cause anxiety or depressions
- be responsible for muscle cramps
- make it more susceptible to infections
- cause chronic fatigue
Many of these ailments stem from the fact that too high acidity blocks essential minerals.
- Teeth
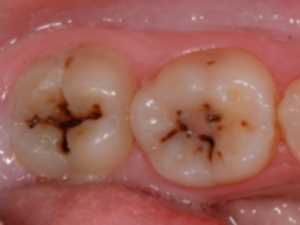 Dental caries is due to the bacteria which decompose sugar by forming acids. They attack the tooth enamel. Bacteria proliferate especially in dental plates which are difficult to remove with a toothbrush .
- Cosmetics
Dental caries is due to the bacteria which decompose sugar by forming acids. They attack the tooth enamel. Bacteria proliferate especially in dental plates which are difficult to remove with a toothbrush .
- Cosmetics
 The natural $pH$ of the skin is between 4.5 and 5.5. Unbalanced ointments can cause skin diseases and early ageing.
The $pH$ of healthy hair is between 4 and 7. Many shampoos are too basic and eventually cause too dry hair.
- Stones
The natural $pH$ of the skin is between 4.5 and 5.5. Unbalanced ointments can cause skin diseases and early ageing.
The $pH$ of healthy hair is between 4 and 7. Many shampoos are too basic and eventually cause too dry hair.
- Stones
 The acidity of rainwater dissolves limestone and causes chemical erosion, particularly spectacular in the caves stalactites and stalagmites.
The acidity of rainwater dissolves limestone and causes chemical erosion, particularly spectacular in the caves stalactites and stalagmites.
 - Dairy products
- Dairy products
 The $pH$ of the milk is approximately equal to 6.8. Lower values may indicate an infection of cattle.
To make cheese, $pH$ is adjusted whether soft or hard cheese is desired (hardness decreases from$pH$ 4-7).
For the production of butter, it is necessary to adjust the$pH$ of the cream to 6,8 strictly.
- The Aquarium
The $pH$ of the milk is approximately equal to 6.8. Lower values may indicate an infection of cattle.
To make cheese, $pH$ is adjusted whether soft or hard cheese is desired (hardness decreases from$pH$ 4-7).
For the production of butter, it is necessary to adjust the$pH$ of the cream to 6,8 strictly.
- The Aquarium
 Most fish can live with $pH$ values between 6.0 and 7.4. However, tolerance depends on their origin, for example fish from equatorial waters prefer water from $pH$ 6.0 to 7.5, cichlids from lakes of eastern Africa with a $pH$ of 7.5 to 8.5.
- The pool
Most fish can live with $pH$ values between 6.0 and 7.4. However, tolerance depends on their origin, for example fish from equatorial waters prefer water from $pH$ 6.0 to 7.5, cichlids from lakes of eastern Africa with a $pH$ of 7.5 to 8.5.
- The pool
 The $pH$ of pool water should be between 6.8 and 7.6. Too high a $pH$ reduces the effectiveness of disinfectants, too low a value irritates mucous membranes.
- The drinking water
The $pH$ of pool water should be between 6.8 and 7.6. Too high a $pH$ reduces the effectiveness of disinfectants, too low a value irritates mucous membranes.
- The drinking water
 The $pH$ of drinking water should be between 6.5 and 8.5. Too acidic water attacks metal pipes, too basic water causes deposits that clog drains.
- Engine
The $pH$ of drinking water should be between 6.5 and 8.5. Too acidic water attacks metal pipes, too basic water causes deposits that clog drains.
- Engine
 Oils for refrigeration engines often become acidic during use. The acid attacks the motor and causes costly repairs.
Oils for refrigeration engines often become acidic during use. The acid attacks the motor and causes costly repairs.