





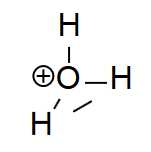
Click on the image to see the ion from other angles
The hydronium ion is present in the aqueous environment. Its molarity is related to the molarity of the hydroxide ion by the ionic product of water:
$[OH^-][H_3O^+]$ $=$ $10^{-14}\frac{mol}{L} $
In acid medium: $[H_3O^+]\gt10^{-7}\frac{mol}{L}$, so $[OH^-]\lt10^{-7}\frac{mol}{L}$ In neutral medium: $[H_3O^+]=10^{-7}\frac{mol}{L}$, so $[OH^-]=10^{-7}\frac{mol}{L}$ In basic medium: $[H_3O^+]\lt10^{-7}\frac{mol}{L}$, so $[OH^-]\gt10^{-7}\frac{mol}{L}$