





The half-life time T1/2 of a radionuclide is the time after which half of a given number of atoms of the radionuclide will be decomposed.
Example
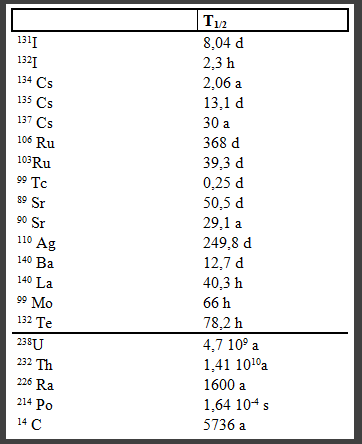
From 100 atoms $^{89}Sr$ will remain 50 atoms intact after 50 days, whereas from 100 atoms $^{140}Ba$, the fact remains that about only 6 remain intact.
The activity of a radionuclide is the number of decays that occur in a sample of single radionuclide per unit time.
The activity is proportional to the number of atoms of the radionuclide. Indeed, more there will be atoms, obviously more there will be breakdowns in the second considered. It is inversely proportional to its half-life. Indeed, the longer the half-life, the less radioactive atoms will tend to break down in the second considered.
The SI unit of activity is the Becquerel ($1\;Bq\;=\;1\;s^{- 1}) $. It is the activity of a sample that provides a radionuclide decay per second.
Example
$1\;g$ of $^{226}Ra$ produced $3.7\cdot10 ^{10}$ decomposition per second. Its activity is $3.7\cdot10^{10}\;Bq\;$ $(=$ $1Ci$ Curie old unit)
The mass activity of a radionuclide is the number of decays that occur per unit mass of the single radionuclide per unit time.
The mass activity is constant for a given radionuclide. We use the unit $\frac{Bq}{kg} $ or quite often the old unit $\ frac{Ci}{g}$.
Example
For $^{226}Ra$ , the mass activity is obviously worth $ 1\frac{Ci}{g}$, for $^{238}U $, it is eg $ 3.2\cdot10^{- 7}\frac{Ci}{g}$, ie $1\; g$ of radionuclide emits $3.2\cdot10^{-}7\cdot3.7\cdot10^{10}$ $=$ $11.840$ decompositions per second.
a) Primordial radionuclides They are radionuclides whose life is long enough that they still persist since the formation of our solar system Eg $^{238}U$ or $^{232}Th$ . They are found in rocks (mainly granitic in the Alps, Vosges, Scandinavian countries, but also shale: Oesling) b) The radioactive decay of primordial radionuclides They are members of different families as natural radioactive $^{214}Po$ that comes from radioactive decomposition originating from $^{238}U $. They are found mainly in the rocks, but some also in the waters and in the atmosphere. c) The radionuclides formed by the effect of cosmic radiation The Earth is constantly bombarded by particles (mostly protons) from the sun or the galaxy. These particles bombard non-radioactive nuclei of our planet and can turn them into radionuclides. Thus $^{14}C$ is constantly formed by bombardment of $^{14}N$. Therefore we will find these radionuclides mainly in the outer atmosphere. d) The radionuclides from nuclear tests and nuclear reactor accidents These radionuclides are generally lighter (the beginning of the first table of this chapter lists those of the radioactive cloud from Chernobyl)
The radiation from radionuclides can be external : - Houses built from granite or slate materials radiate more than some houses built of sandstone, limestone or precast. - Depending on the origin, plaster $CaSO_4$ can be more or less contaminated with radioactive radium sulfate $RaSO_4$ . - Passenger aircrafts are subjected to more intense radiation which varies according to latitude, solar cycles and generally increases with altitude. - Coal or phosphate rock radiate strongly enough because of the presence of many radionuclides. - Phosphate fertilizers are often irradiating due to the presence of ${226}^Ra$ . - Watches and radioluminescent dials are radioactive because they use radionucléides to maintain luminescence. - Lots of ceramics, enamels or optical glasses are irradiating since they use uranium or thorium. Internal Irradiation occurs whenever there is ingestion of radionuclides:: - Radionuclides by breathing air. - Radionuclides by water sources of granitic regions or water polluted rain (thunderstorm rainfall of 3 May 1986 in Luxembourg) which then pass in the fish of rivers. - Radionuclides present in grass which is watered by rain, which then passes to contaminated milk products or to the game - Radionuclides in fruits, mushrooms and vegetables also contaminated from rainfall . Irradiation is continued if the radionuclide is incorporated into a biochemical molecule (eg $^{131}I $ in the thyroxine, thyroid gland hormone).
Radionuclides project particles (alpha or beta;) or emit gamma rays. According to their specific activities, but also according to the transmission speed of the particles or the frequency of their radiation, the total energy emitted by radionuclides in a given time can vary greatly. To assess possible effects of such radiation, it is particularly interested to consider the received energy (Eg by man or a particular sensitive tissue as the gonads.)
The absorbed dose is the energy emitted by radionuclides unit mass of the irradiated areas. The SI unit of absorbed dose is Gray ($1\;J/kg$ $=$ $1\;Gy$ $=$ $100\;rad$). An irradiated medium receives a dose of 1 Gray, if each kilogram absorbs 1 Joule energy due to irradiation.
The absorbed dose is calculated knowing the radionuclide activity to which humans are exposed (a factor of dose is taken into account for each radionuclide according to its virulence), their concentration in the medium and the radiating exposure time.
Example
Because of the Earth's radiation, Switzerland receives an hourly dose of 7.4 mrad, while a Hindu receives only 3.6
The radiation may alter the DNA and therefore the hereditary code of the cell . In the case of a somatic cell, different cases are possible: a) the synthesis of proteins essential for cellular metabolism is no longer possible, the cell dies. b) the synthesis of proteins essential to ensure the proper cell division and transmission of code to daughter cells is not possible: The daughter cells will become unsustainable, they die. c) the anomaly produced on DNA causes cell mutation that is passed on to descendants. This change causes for example an increase in the frequency of dividing the cell line in question, a benign or malignant tumor may develop. In the case of a germ cell, besides the cases a) and b) above, the offended DNA transmitted to the offspring can cause impairment in children to be dominant or recessive according to the DNA portion affected.
The simple absorbed dose often not realizes the severity of the risk. One may for example receive the same dose as a result of ingestion of iodine or radioactive radon, but the iodine represents the greatest risk because it will be incorporated into the thyroid, a particularly sensitive organ. It is therefore necessary to correct the absorbed dose by factors that reflect the seriousness of this absorption.
The biological dose measurement is called the equivalent dose . It is expressed in Sievert ($Sv$) Most commonly used is the $rem$ ($1\; Sv$ = $100\; rem$) "Radiation Equivalent Man". This unit is obtained by multiplying the absorbed dose ($rad$) by two factors; the quality factor (QF) and the distribution Factor (DF) . We therefore have: ED (rem) = D (rad) x FQ x FD
The quality factor is taken arbitrarily to 1 for photons and electrons and 10 for the most destructive alpha particles. The distribution Factor is 1 when dealing with radionuclides uniformly distributed in the body such as $^{40}K$, 5 for radionuclides irregularly distributed in the body such as $^{131}I$.
Orders of magnitude:
| 1 mSv | Chest x-ray |
| 2 mSv | Natural Irradiation average in France |
| 5 mSv | External limit exposure of the population |
| 50 mSv | External limit exposure of workers of category A (radiation work) of the nuclear industry |
| 0.3 Sv | Spontaneous reversible change in the blood |
| 1 Sv | Hospitalization for the health check (whole body exposure) |
| 4.5 Sv | Lethal Dose 50 (50% mortality) |
| 6 Sv | Erythema (local irradiation) |
| 40-80 Sv | Dose used in radiotherapy |
- The interior of the Earth contains radioactive elements that produce heat, and this heat is removed by convection currents in the mantle and conduction in the lithosphere. The movements of the mantle surface cause the large displacements of the substantially rigid tectonic plates. That is the source of volcanism, earthquakes and tsunamis.

- The exposure of germ cells to the radioactive radiation produces mutations . These mutations are responsible for the evolution of species by natural selection.

Image Karen Carr