







Calculate the percentage of the ionization (%) of a $0.400\;M$ solution of $HN_3$
(hydrazoic acid, $K_a$ $=$ $1.9\cdot 10^{-5}$)
Equilibrium of hydrolysis:
$HN_3$ $+$ $H_2O$  $H_3O^+$ $+$ $N_3^-$
$H_3O^+$ $+$ $N_3^-$
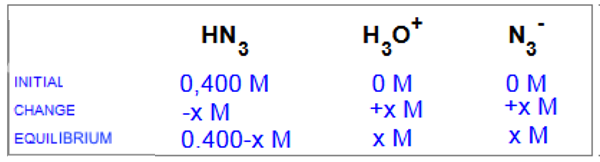
$K_a$ $=$ $1.9 \cdot 10^{-5}$ $=$ $\frac{x^2 }{0.400-x }$ After having determined $x$ $=$ $2.8 \cdot 10^{-3}\;M$, how do we calculate the % age of ionization starting with $x$ $=$ $[H_3O^+]$?
a)% ionisation = $\frac{[H_3O^+]_{equilibrium}}{[HN_3]_{initial}}\cdot 100$ b)% ionisation = $\frac{[HN_3]_{initial}}{[HN_3]_{equilibrium}+[H_3O^+]_{equilibrium}}\cdot 100$ c)% ionisation = $\frac{[HN_3]_{equilibrium}+[H_3O^+]_{equilibrium}}{[HN_3]_{initial}}\cdot 100$ d)% ionisation = $\frac{[H_3O^+]_{equilibrium}}{[HN_3]_{equilibrium}+[H_3O^+]_{équilibrium}}\cdot 100$
a)% ionisation = $\frac{[H_3O^+]_{equilibrium}}{[HN_3]_{initial}}\cdot 100$ $ =$ $0.70 $%