







You dispose of a $0.200\;M$ solution of $H_3C_6H_5O_7$
(citric acid, $K_a$ $=$ $3.5\cdot 10^{-4}$)
Equilibrium of hydrolysis:
$H_3C_6H_5O_7$ $+$ $H_2O$ $H_3O^+$ $+$ $H_2C_6H_5O_7^-$
$H_3O^+$ $+$ $H_2C_6H_5O_7^-$
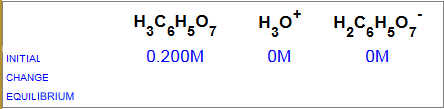
What are the changes in concentration of each species?
a) $+x\;M$, $-x\;M$, $+x\;M$ b) $-x\;M$, $+x\;M$, $+x\;M$ c) $+x\;M$, $+x\;M$, $+x\;M$ d) $-x\;M$, $+x\;M$, $-x\;M$
The answer b) is correct, because when one mole of acid dissociates, one mole of each ion appears.