






Willing to test Thomson's "plumpudding" atomic model, Ernest Rutherford imagined to bombard gold atoms with α particles obtained by radium. Indeed, knowing that these particles are quite heavy and charged positively, he wanted to test if the positive "heart" of the gold atoms would let them pass or no. Under no circumstances, α particles could be deviated by electrons which are much too lightweight. (a cannonball is not deviated by a fly!).
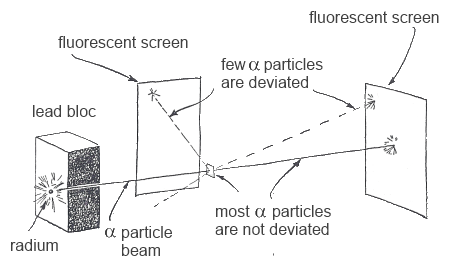
The experiment was realised in the laboratory of Rutherford and did not go like expected, only few α particles were deviated :

The few deviated α particles must have been thrown out of their trajectory by
A very small and heavy mass situated at the heart of the atom: the nucleus All the rest of an atom is void except for the presence of the electrons: the electron cloud
The electron cloud permits the passage of α particles without deviation.